
At this very moment, every pool of water on the planet—from the Pacific Ocean to the puddle in your driveway—is undergoing an invisible, inexorable change: It is becoming more acidic. Every day, human beings pump 21 million metric tons of carbon dioxide into the Earth’s atmosphere from the burning of fossil fuels. Much of that CO2 is absorbed by water, producing a substance called carbonic acid. Nowhere is this more true than in the oceans.
This process, called ocean acidification, is changing the oceans more drastically and more quickly than at any other time in the last 60 million years. In other words, the last time the world’s oceans saw such a rapid and significant change in acidity was just after the demise of the dinosaurs. This change has already begun to wreak havoc with marine species that depend on the delicate chemical balance of ocean water for their own survival. What’s more, it’s thrown into question the future of many people whose livelihoods depend on the sea.
Mark Green, a professor of marine science at St. Joseph’s College who grows Basket Island oysters at his own shellfish company in Casco Bay, spends a great deal of time thinking about ocean acidification. “All marine shells and coral reefs are made out of calcium carbonate,” says Green, “and that’s what will dissolve in order to neutralize the acid. That’s the Tums and Rolaids of the ocean.”
When acidic seawater dissolves its buffer, existing corals and shells are destroyed. The concentration of carbonate ions in the ocean decreases sharply. Unfortunately for shellfish—not to mention the people who harvest and eat them—carbonate ions in the seawater are necessary for all shell-forming animals and corals to grow in the first place.
In addition to the global problem of carbon emissions, Green points out that ocean acidification has another significant source, one that is a particular problem for populated coastal areas such as the Gulf of Maine: runoff from storm water and wastewater treatment that is rich in nitrate. That nitrate, like fertilizer in garden soil, causes a dramatic increase in the growth of photosynthetic organisms—plants, phytoplankton, and algae—in shallow water. When those organisms die, they release their absorbed CO2, acidifying the surrounding water and especially the mud on the bottom. The amount of dissolved oxygen in the surrounding water is also decreased by decomposition, leading to so-called “dead zones.”
A soft winter rain patters onto the gray metal roof of a Quonset hut at the end of a long driveway on a hill, overlooking the Damariscotta River. Aside from the parked pickup trucks and piles of snow-covered plastic mesh, there is little evidence that this is a thriving aquaculture business.
Bill Mook pulls up in a slightly dented truck and strides through the door. Inside, he moves through a warren of Sheetrocked walls, past several open tanks of pale-green, filtered river water to a warm, modest office overlooking the river.
While doing graduate work in oceanography at the University of Maine, Mook took a job at an oyster hatchery, and a few years later, launched his own company, Mook Sea Farm. He and his eight employees grow and sell 70 to 80 million oysters a year, mostly as seed for other oyster growers up and down the East Coast. In the summer, they shift their labor out of the hatchery and onto the river, where they grow their own oysters to market size.
From his office, it’s a short walk down the hallway to the laboratory. Inside, a colleague uses a microscope to examine a sample of microalgae. The orange liquid—food for the millions of larval oysters in the building—is one of several strains that Mook grows himself in an adjoining room, in clear plastic jugs nestled against a wall lit from within by fluorescent lamps. The jugs bubble with compressed CO2, which is added through a system of plastic tubing. Microalgae, like plants, consume CO2 and produce oxygen. They look like boiling bottles of organic fruit juice, or perhaps a kombucha experiment gone horribly awry.
Downstairs, the environment is completely different. It’s steamy and warm, faintly yeasty, like a brewery. Technicians move trays of oyster larvae floating in water with pulverized shell. What looks like slightly dark sand, or river silt, is actually millions of oysters, building shells, consuming microscopic algae, and steadily growing. Large tanks hum. Pumps cycle on and off.
But something is changing. Mook and his team have noticed a sharp drop over the past few years in the amount of microalgae present in the river water. More worrisome still, they have had greater difficulty using the water to grow certain cultures of algae.

Damariscotta River Photo: Jason Mann
“There’s clearly higher dissolved CO2 in the water,” Mook says, and he suspects that may be partially to blame.
Among his oysters there is another noticeable (though as yet nonlethal) effect. Oyster larvae normally spend their first few weeks of life swimming around, swarming in various corners of the tank, and moving up and down in the water column. After those first 10 days or so, the oysters develop “eye spots,” photosensitive sites that enable them to sense the bottom and swim toward it. There, they latch on to bits of discarded and crushed shell.
Mook’s oysters, though, have exhibited a strange behavior: At certain times, particularly after runoff from heavy rains has added more freshwater to the river—water that is more acidic—his larval oysters have clustered in the bottom of the tank, motionless.
Many species of clams, oysters, and mussels—especially in their larval stage—appear to be growing more fragile in the conditions Mook has observed in his hatchery. Whether they’ll survive may depend on how much conditions in the East resemble those in the Pacific Northwest.
“We never used to think about water chemistry at all unless we had to,” says Alan Barton, speaking on a scratchy cell phone from the Whiskey Creek Shellfish Hatchery in Tillamook, Oregon. “The only thing we knew to care about was bacteria and diseases. We never thought about chemistry at all.”
In late 2007, the hatchery began to have significant production problems. Since 1978, it had been a remarkably consistent and successful operation. But suddenly, batches of larvae began dying off. Production at the hatchery, which at roughly 10 billion oysters a year is one of the three main suppliers to the entire Pacific Northwest oyster industry, fell by 75 percent.
Then, one day in July 2008, everything stopped.
“Everything was on the bottom,” Barton says. “Basically every oyster in the hatchery died in one day. We just had to put on the brakes.” That day, the workers at Whiskey Creek began to zero in on acidic water as the cause. They began to buffer the hatchery water with sodium bicarbonate, raising the pH. The larval oysters responded immediately. They had found the culprit.
Off the coast of Oregon, water from the depths of the Pacific Ocean has always moved up to the surface in a recycling process called upwelling. Deep ocean water is frequently more acidic because organisms decay as they sink, but something else has changed.
“Water from the deep ocean always has been more acidic,” Barton says. “We get a devastating smack in the face from upwelling events. But it’s not upwelling season and we’re buffering the water right now. We’ve been buffering since we started this season.”
The system at Whiskey Creek adds roughly the same amount of buffering to the water as the amount by which ocean scientists say the global acidity of the ocean has increased. With the help of sophisticated monitoring equipment and the assistance of ocean scientists, Barton is convinced that the increase in human-derived CO2 in ocean water is the source of their production problems. And it frustrates him that the story of what happened at Whiskey Creek—the big die-off, the increase in monitoring, the precise buffering of the water, the tentative recovery—has largely been given a happy ending.
“You hear that we saved the industry and fixed it and it’s all better now,” says Barton. “But that is not true. Yes, it’s better because we’re monitoring and controlling carbon chemistry. But we work a lot harder than we used to.
“We are already past the threshold at which many of these species can live and reproduce. Ultimately, the carbon chemistry of the world’s ocean has changed.
“It’s all slipping away from us.”
It’s 8 a.m. on a Thursday morning in a hotel meeting room in Rockport, and the annual Maine Fishermen’s Forum has yet to start. Even so, 60 fishermen, scientists, reporters, and fishing industry regulars have gathered to hear a panel of speakers on the issue of ocean acidification and its effect on fisheries. Mark Green and Bill Mook are there, as are scientists from the University of Maine and NOAA, a clammer, and Dave Cousens, the president of the Maine Lobstermen’s Association. It’s a good turnout. There are even some laughs, such as when the moderator claims that a Republican Senate staffer in D.C. told him on a recent visit that he was forbidden to use the words climate or change under any circumstances.
Mark Green leads off the Fishermen’s Forum panel discussion with what he calls “Ocean Acidification 101.”
“This is the single biggest environmental problem on the planet,” says Green. “Even though we’ve put 500 billion tons of CO2 into the ocean, just an insane quantity, there’s more than enough buffer there.” But that buffer is the calcium carbonate shells of clams, oysters, and mussels, and corals. Green points out that the change in ocean acidity is not just potentially disastrous, it’s also happening at a frighteningly rapid pace.
“We’re injecting CO2 into the ocean much, much faster, at least 100 times faster, than has ever occurred before,” Green says. The amount of acid in the oceans has increased by 30 percent in just the last 50 years. Though some of the creatures in the ocean can adapt to survive in more-acidic water, evolution will likely be far slower than the pace of acidification that humans have set.
They discuss possible mitigation strategies, such as covering clam flats with crushed shell to buffer them from the increased acidity. Then the talk turns toward the future and the political challenges it portends.
“We are starting to see several of the indicators on the East Coast that we saw in the West,” Green says. Mook nods in agreement.
“There is a gathering storm headed our way,” Mook says. “It’s really high time we started paying attention.”

What effect this drastic change in ocean chemistry will have on Maine’s most lucrative and important fishery, lobsters, remains unknown. There’s simply no clear answer yet.
According to Green, “We know that there will be some winners and losers; pH is an indicator, not a magic ball.” Green crabs, for example, seem to be surging in population. It’s quite possible that as the shells of clams become thinner, they will make easier prey for crab species. What happens to those species as their populations surge in opposite directions is unclear. But the scientists and fishermen alike seem to agree that it won’t be good. It’s just one more significant stressor on animals dealing with conditions, including a warming ocean, that are unlike any in recorded history.
Cousens, a lifelong lobsterman, has heard enough.
“I’ve seen drastic changes in the lobster industry,” he says, his voice rising. In the audience, spines straighten and side conversations pause. “You used to be able to set your clock on when the shedders would arrive. July 17 to 19—that was it. Now they’re coming all year-round. It’s scary.”
He is just warming up.
“These politicians are morons! When they see a crowd of pissed-off fishermen in their office, then they’ll do something about climate change!”
The room applauds.
“We have to lower our production of carbon,” says Green matter-of-factly. “There’s a hundred different ways to spin a turbine. For whatever reason, we still do it by lighting things on fire.”
In his office, I ask Bill Mook about mitigation. Does it imply that we’re giving up on fixing the root cause of the problem?
“I think we’re focused on the things we can do while people fix or don’t fix the larger carbon problem,” he says. “Most of these steps would be good to take ecologically anyway.”
But is there a sense of urgency among the oyster growers he knows regarding ocean acidification?
“No,” he says. “Look, at hatcheries, we’re the first line of defense. Every time we change the water in our larval tanks, we’re measuring the water quality. So we see it immediately. One of the big jobs I see is to convince people that it’s going to affect things.
“People like Dave Cousens ranting at the Fishermen’s Forum can have a much bigger effect on fishermen than a scientist talking about monitoring.”
Ocean acidification hovers just below the public consciousness now, a source of specific worry for some, perhaps of vague dread for a few more. But it is exploiting the psychological weak spot between our ability to plan for the inevitable and our willingness to fight a problem head-on. Right now, apart from a handful of oyster growers and scientists, Americans are doing neither. Of all the side effects of human reliance on fossil fuels, it may be the simplest to predict scientifically, and—in concert with warming ocean temperatures and increasingly intense storms—one of the most directly damaging for coastal communities around the world. Yet it remains virtually unknown among the general public.
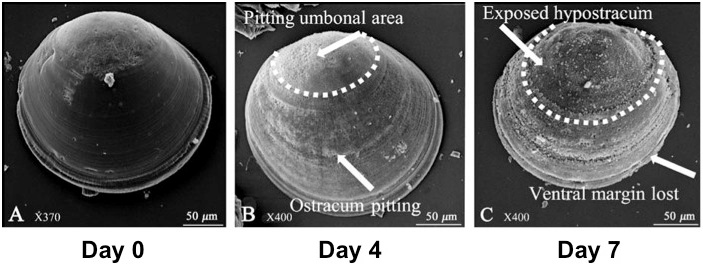
“I don’t want to spend my time telling people about this problem,” says Alan Barton, “but I do it. I talk about this, because I feel like we have this big secret.”
Regardless of action on human carbon emissions, there is significant change coming for the world’s oceans. Even in the best-case scenario, acidification will continue for the foreseeable future. The ability of marine ecosystems to respond to this stress is largely, with the exception of specific areas like clam flats, beyond human control. Swift action to curb carbon emissions would affect the long-term result of acidification—just how bad the situation gets—but the next several decades will likely see increasing acidity in the ocean no matter what happens on land.
On the other hand, if we are able to control the other stressors that affect marine species—preventing algal overgrowth by preserving herbivorous fish species, for example, and decreasing the nutrient-rich runoff from stormwater—we may give those animals a fighting chance.
Bill Mook considers this. The clouds over the river have partedmomentarily. The omnipresent dripping of the late-winter thaw taps on the corrugated roof. The river rushes on.
“I still feel basically optimistic about this business. Before, we were so engrossed in the day-to-day. Now we’re able to think about what’s coming down the road. I still see a lot of opportunity. And we’re going to do everything we can to survive.”




